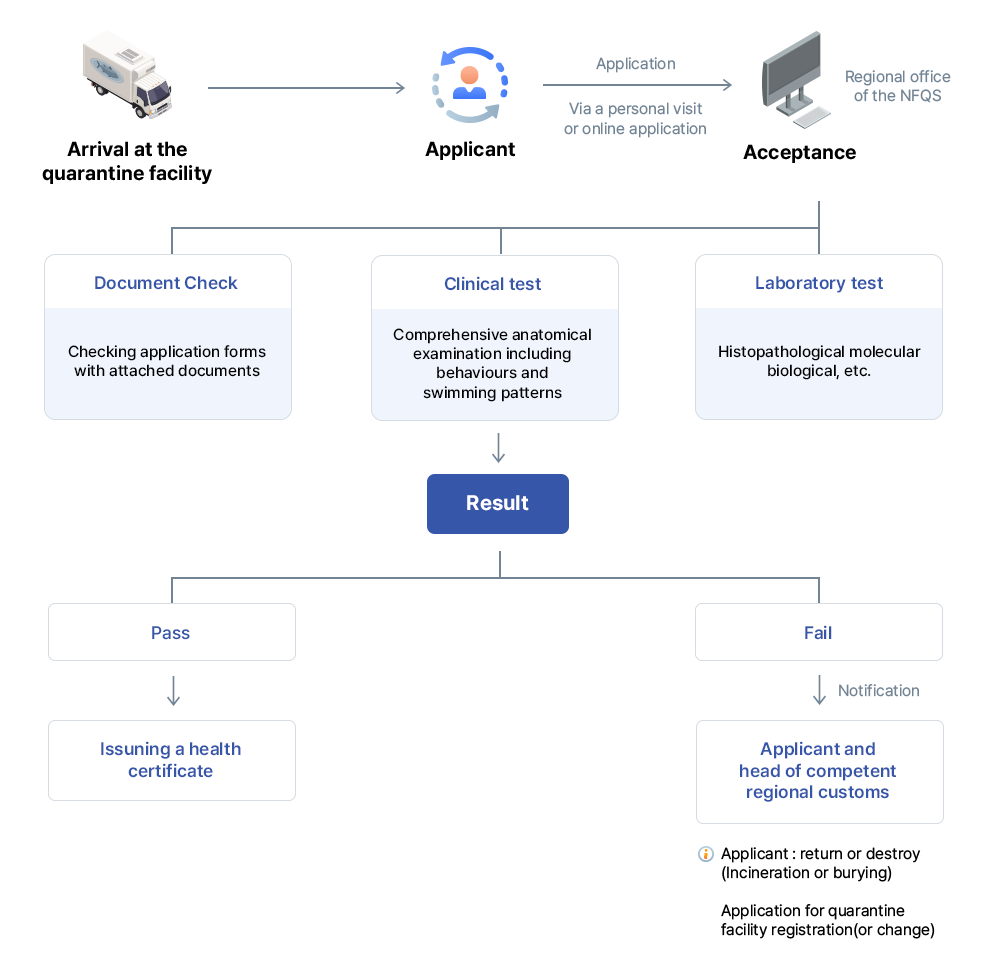
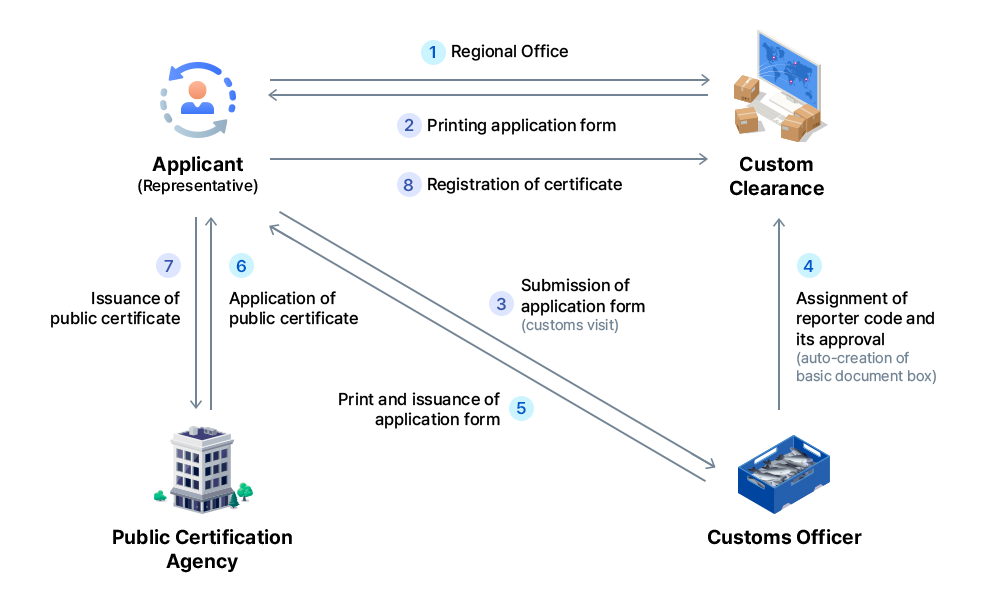
Legal basis - Aquatic Organism Disease Control Act
-
Article 22Quarantine of Exported and Imported Aquatic Organisms
-
Article 23Designed Quarantine items
-
Article 27Quarantine Insptection on Import
-
Article 31Quarantine Insptection for Export